The JEE Main exam tests your understanding of fundamental physics concepts, and thermodynamics is a crucial chapter you need to conquer. This branch of physics deals with the relationship between heat, work, temperature, and energy. Mastering thermodynamics equips you with the tools to analyze various processes involving energy transfer and transformation. Let’s delve into the key concepts and equip you for success!
Basic Definitions
- Thermodynamic System: A portion of the universe that you’re interested in studying. It can be a closed system (no exchange of matter with the surroundings) or an open system (allows exchange of matter).
- Surroundings: Everything outside the system that interacts with it through heat and work transfer.
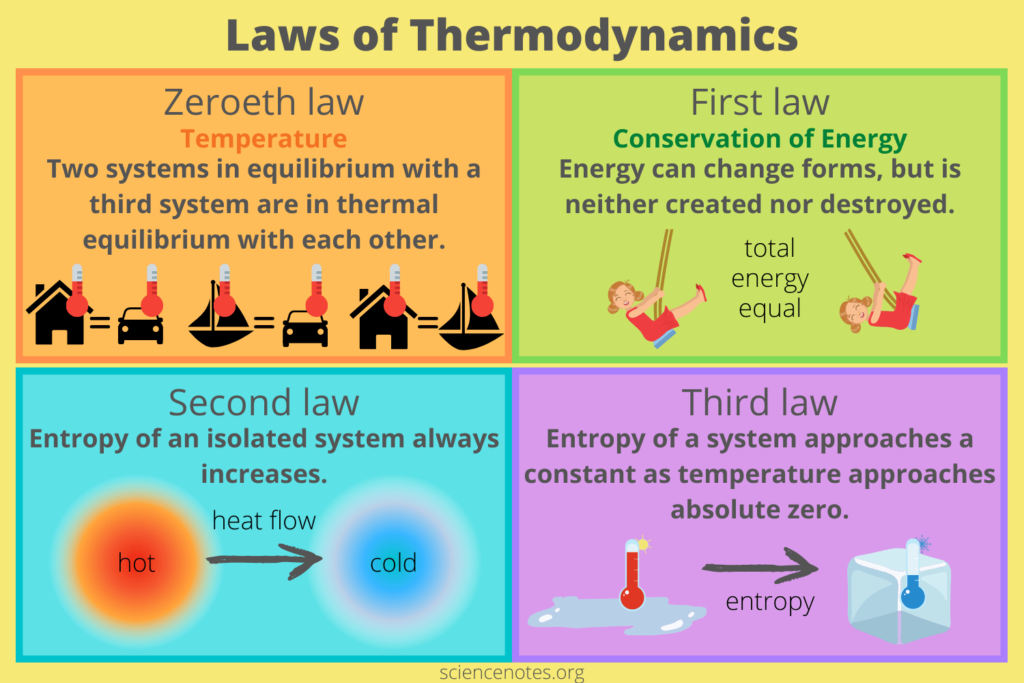
- Thermodynamic Equilibrium: A state where macroscopic properties (temperature, pressure, volume) don’t change with time. Both the system and surroundings are in thermal equilibrium when there’s no net heat transfer between them (Zeroth Law of Thermodynamics).
Thermodynamic Properties
- Temperature (T): A measure of hotness or coldness, often measured in Kelvin (K).
- Pressure (P): Force per unit area exerted by a gas on its container walls, typically in Pascals (Pa).
- Volume (V): The space occupied by a substance, measured in cubic meters (m³).
- Internal Energy (U): The total kinetic and potential energy of the particles within a system.
Thermodynamics Laws
- First Law of Thermodynamics (Law of Conservation of Energy): The total energy of an isolated system remains constant. Heat (Q) transferred to a system increases its internal energy, and work (W) done by the system decreases its internal energy (ΔU = Q + W).
- Second Law of Thermodynamics (Law of Increased Entropy): In any spontaneous process, the entropy (S) of the isolated system and its surroundings always increases. Entropy is a measure of randomness or disorder in a system. This law implies that some processes, like complete conversion of heat into work, are irreversible.
- Third Law of Thermodynamics: The entropy of a system approaches a constant minimum value as its temperature approaches absolute zero (0 K).
Thermodynamic Cycles
Thermodynamic processes describe changes in the state of a system. Some important ones include:
- Isothermal Process: Temperature remains constant (ΔT = 0).
- Adiabatic Process: No heat transfer occurs between the system and surroundings (Q = 0).
- Isobaric Process: Pressure remains constant (ΔP = 0).
- Isochoric Process: Volume remains constant (ΔV = 0).
Thermodynamic cycles involve a series of connected processes that return the system to its initial state. The Carnot cycle is a theoretical, reversible cycle that sets the upper limit for the efficiency of heat engines.
Applications of Thermodynamics
Thermodynamics has diverse applications beyond theoretical understanding. Here are a few examples:
- Heat Engines: They convert heat into work, powering everything from cars to power plants. Understanding thermodynamics helps optimize engine efficiency.
- Refrigerators and Air Conditioners: These devices operate on the principle of reverse heat engines, removing heat from a cold reservoir to maintain a lower temperature.
- Chemical Reactions: Thermodynamics helps predict the spontaneity and feasibility of chemical reactions based on enthalpy changes (heat transfer at constant pressure) and entropy.
Practice Makes Perfect: Tips to Ace Thermodynamics in JEE Main
- Grasp the fundamentals: Ensure a clear understanding of basic concepts like heat, work, and internal energy.
- Master the laws: Apply the first, second, and third laws of thermodynamics to analyze various processes.
- Practice with different process types: Solve problems involving isothermal, adiabatic, isobaric, and isochoric processes.
- Focus on cyclic processes: Understand the concept of thermodynamic cycles and their applications.
- Solve previous year’s questions: Analyze past JEE Main papers to understand the type and complexity of questions asked.
- Seek guidance: Don’t hesitate to clarify doubts with teachers or mentors.
Thermodynamics may seem daunting at first, but with focused study and practice, you can master this crucial chapter for JEE Main. Remember, a strong foundation in these concepts will not only help you excel in the exam but also equip you to tackle advanced physics concepts in the future. So, buckle up, embrace the challenge, and conquer the world of thermodynamics!
Additional Resources:
- NCERT Physics Textbook (Class XI & XII)


